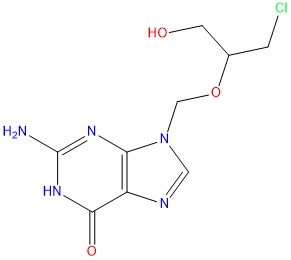
Purity testing of Ganciclovir EP Impurity C ensures that it meets stringent quality criteria, guaranteeing its suitability for accurate analytical assessments and research applications.
Solubility studies are crucial to evaluate how effectively Ganciclovir EP Impurity C dissolves in various solvents, aiding in its formulation and integration into pharmaceutical processes.
Stability assessments examine the chemical resilience of Ganciclovir EP Impurity C under diverse environmental conditions, ensuring consistent reliability over time for pharmaceutical development and manufacturing practices.
Ganciclovir EP Impurity C is synthesized in compliance with Good Manufacturing Practice (GMP) guidelines by experienced chemists using advanced equipment and analytical methodologies.
- CAT NO : AE-00017
- CAS No : 108436-36-8
- Mol.F. : C9H12ClN5O3
- Mol.Wt. : 273.7
- Inv. Status: In Stock
- Technical Certificate