The synthesis of Anastrozole EP Impurity D involves multi-step organic reactions starting from commercially available starting materials.
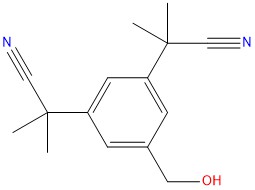
The purity of Anastrozole EP Impurity E is guaranteed to be equal to or greater than 98%, ensuring its suitability as a reliable reference standard for analytical testing and research purposes.
Anastrozole EP Impurity E is synthesized according to Good Manufacturing Practice (GMP) guidelines by experienced chemists using state-of-the-art equipment and analytical techniques.
- CAT NO : AE-00016
- CAS No : 120511-88-8
- Mol.F. : C15H18N2O
- Mol.Wt. : 242.3
- Inv. Status: In Stock
- Technical Certificate