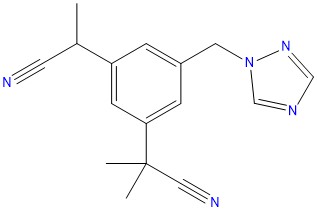
Abacavir EP Impurity A is synthesized according to Good Manufacturing Practice (GMP) guidelines by experienced chemists using state-of-the-art equipment and analytical techniques.
The purity of Abacavir EP Impurity A exceeds [specified percentage], ensuring its reliability as a standard for precise analytical testing and research applications.
Stability testing of Abacavir EP Impurity A confirms its resilience under specified storage conditions, ensuring consistency and reliability for analytical purposes.
- CAT NO : AE-00007
- CAS No : 1215780-15-6
- Mol.F. : C16H17N5
- Mol.Wt. : 279.3
- Inv. Status: In Stock
- Technical Certificate