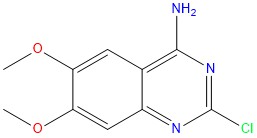
The purity of Alfuzosin EP Impurity B, exceeding 98%, ensures its reliability as a standard for accurate analytical testing and research applications.
The solubility of Alfuzosin EP Impurity B has been observed to be higher in organic solvents such as methanol, ethanol, and dimethyl sulfoxide (DMSO), with limited solubility in water.
Stability studies have confirmed that Alfuzosin EP Impurity B maintains its chemical integrity under recommended storage conditions, ensuring consistency and reliability for analytical testing and research purposes.
Alfuzosin EP Impurity B is synthesized according to Good Manufacturing Practice (GMP) guidelines by experienced chemists using state-of-the-art equipment and analytical techniques.
- CAT NO : AE-00019
- CAS No : 23680-84-4
- Mol.F. : C10H10CIN3O2
- Mol.Wt. : 239.7
- Inv. Status: In Stock
- Technical Certificate