The purity of Abacavir EP Impurity E surpasses industry standards, ensuring its efficacy as a reliable reference material for precise analytical testing and research applications.
The solubility characteristics of Abacavir EP Impurity E have been thoroughly evaluated, demonstrating its effective dissolution in various organic solvents, facilitating accurate analytical procedures and research applications.
Stability testing confirms that Abacavir EP Impurity E maintains its integrity under specified storage conditions, ensuring consistent performance and reliability for analytical testing and research applications.
Synthesized in accordance with Good Manufacturing Practice (GMP) guidelines by experienced chemists utilizing cutting-edge equipment and analytical methodologies.
- CAT NO : AE-00010
- CAS No : 208762-35-0
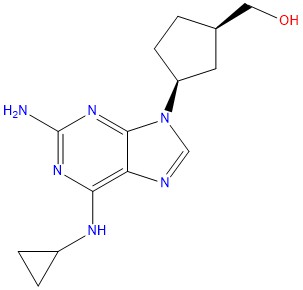
- Mol.F. : C14H20N6O
- Mol.Wt. : 288.4
- Inv. Status: In Stock
- Technical Certificate