Impurities can sometimes affect the efficacy or potency of the active pharmaceutical ingredient. By monitoring impurity levels, manufacturers can ensure that the medication delivers the intended therapeutic effect consistently.
Abacavir EP Impurity A is synthesized according to Good Manufacturing Practice (GMP) guidelines by experienced chemists using state-of-the-art equipment and analytical techniques.
The purity of Abacavir EP Impurity A exceeds [specified percentage], ensuring its reliability as a standard for precise analytical testing and research applications.
Stability testing of Abacavir EP Impurity A confirms its resilience under specified storage conditions, ensuring consistency and reliability for analytical purposes.
- CAT NO : AE-00009
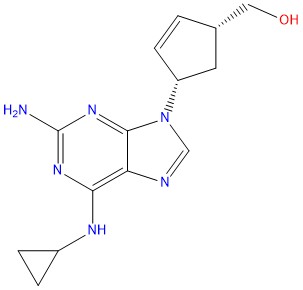
- CAS No : 136470-79-6
- Mol.F. : C14H18N6O
- Mol.Wt. : 286.3
- Inv. Status: In Stock
- Technical Certificate